History of AF Ablation
The first catheter ablation in humans was performed by Dr. Melvin Scheinman in 1981, using high energy DC shocks. Dr Scheinman remains today as an active member of the electrophysiology group at UCSF. Dr Scheinman’s work led directly to the development of radiofrequency energy catheters, which use radiofrequency energy to heat the catheter tip and perform much more precise ablation than was possible with DC ablation. Cather ablation has been used for many years to treat arrhythmias due to a focal source or pathway, such as AV nodal reentrant tachycardia or Wolff-Parkinson-White syndrome. However, only over the past decade has catheter ablation been used to treast more complex rhythms like atrial fibrillation.
In 1998, Michelle Haissaguerre, a cardiac electrophysiologist in Bordeaux, France first described the use of catheter ablation for patients with atrial fibrillation. He put catheter in patients’ hearts and mapped the origin of the “triggers” that start atrial fibrillation. He found that 95-96% of the time these triggers originate in sleeves of muscle that extend into the pulmonary veins (PV), the veins that drain blood from the lungs back into the left upper chamber of the heart (left atrium). By mapping these triggers during the initiation of AF and ablating them within the pulmonary vein, he was able to render 62% of patients free of AF without the need for antiarrhythmic drugs. This landmark finding has led to the development of catheter ablation as a routine management strategy for AF.

Location of AF triggers in 38 patients with AF. From Haissaguerre et al. NEJM 1998.
The initial strategy for catheter ablation of AF was to map and ablate these triggers within the pulmonary vein. Patients were brought to the electrophysiology lab and given adrenaline to try and initiate their AF. If no AF could be initiated patients were sent home. If triggers were initiated the earliest activation point was mapped and ablated.
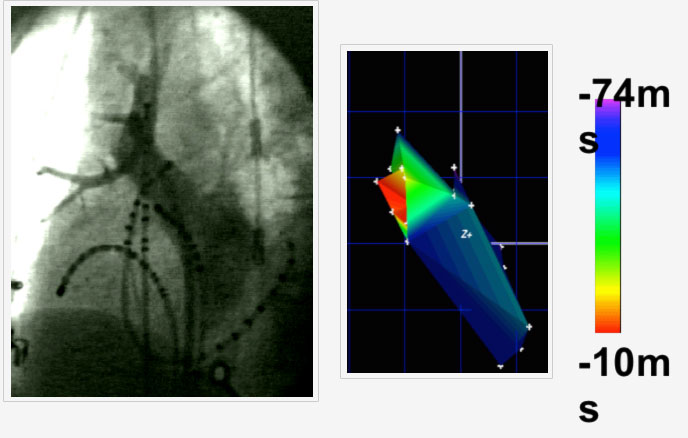
Figure: Pulmonary venogram (left) with multiple catheters in the right upper pulmonary vein (RUPV) The trigger for AF within the PV was mapped and shown in red in the left panel as the site of earliest activation of AF.

Circular mapping catheters used for electrical PV isolation
Although this strategy worked in some patients, it was limited by the inability to induce AF triggers in up to 1/3 of patients, and a high recurrence rate after ablation. In the series published by investigators at UCSF in 2001, long-term success (freedom from AF without drugs 14 mos after ablation) was 33%, with an additional 13% of patients who had no AF, but remained on drug therapy. The other problem with this approach was that ablation distally within the pulmonary vein could sometimes lead to a constriction or stenosis of the pulmonary vein, which could lead to chronic shortness of breath. While treatable with PV “stenting,” it was clear that a better approach was needed.
In 1991, Haissaguerre again described a new approach for PV isolation. Instead of mapping the individual triggers inside the PV, ablation was performed more proximally where the PV inserted into the left atrium. By using a circular catheter with multiple electrodes on it, one could localize the muscle sleeve connections and electrically disconnect the entire PV from the left atrium. This effectively prevented any triggers inside the PV from starting AF, even if they could not be provoked in the electrophysiology laboratory. Thus, the procedure of empiric 4 PV disconnection for treating AF was born.
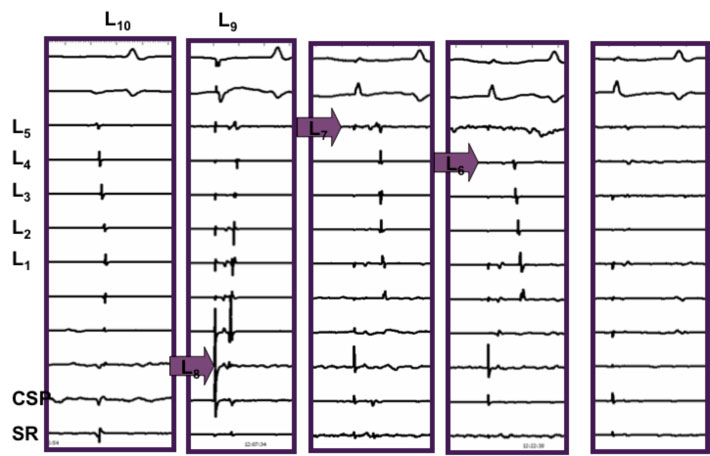
Electrical disconnection of the PV by ablating the site of earliest activation at the PV os using recordings from the circular mapping catheter (L).
Since 2001, several incremental improvements in the PV isolation approach have been developed. The location of PV isolation has moved more proximally, from the PV ostium to the antral insertion of the PV, several centimeters proximal to the PV ostium. This so called “wide area circumferential ablation” or WACA, also known as PV antral isolation, has several advantages. First, the likelihood of PV stenosis has decreased dramatically. Second proximal AF triggers that may originate from the PV antrum are also eliminated, and third, modification of nerve bundles that innervate the atria and contribute to AF maintenance may also be ablated. Thus the current procedure involves placement of several catheters into the heart, transseptal puncture to cross into the left atrium, and radiofrequency ablation in a “connect-the-dots fashion to proximally isolate the PV antrum. A temperature sensor is typically placed within the esophagus to indicate any heating of the esophagus while ablating in the posterior left. Use of noninvasive imaging of the heart with computed tomography or magnetic resonance imaging can be used to guide ablation. 3D reconstruction of the left atrium can be registered with the catheter mapping system so that the catheter is manipulated inside a 3D reconstruction of the individual patients left atrium. This allows tailoring of the ablation lesions to variation in left atrial size and PV anatomy.
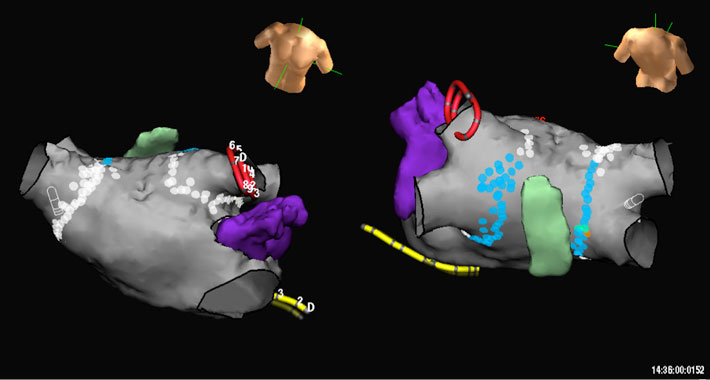
Figure: CT angiogram of the left atrium and esophagus. The structure in purple is the left atrial appendage.The white and blue dots represent ablation lesions around the pulmonary veins. The blue dots represent lower power lesions delivered on the posterior wall of the left atrium when in the vicinity of the esophagus.
In addition to PV isolation, at UCSF we continue to also search for AF triggers outside of the PVs, which may account for up to 5% of AF triggers, more commonly in women. This is performed by giving an infusion of isoproterenol (adrenaline) to try and trigger AF and localize the site of origin. Common sites of origin outside the PVs include the superior vena cava, crista terminals, and mitral valve annulus. In addition occasionally regular rapid rhythms due to common arrhythmias such as AV node reentry, Wolff-Parkinson-White syndrome, or atrial tachycardia may trigger AF. If this is the case, a simpler ablation to address the underlying problem may be all that is required.
This PV antral isolation procedure has been accepted at most centers as the standard for patients with paroxysmal AF. The need for repeat procedures may be higher in patients with more persistent forms of AF. Fibrosis, dilatation and other structural changes occur in the atrium after years of AF and tend to make AF more easily sustained. Premature atrial beats from areas outside the PVs may also initiate AF in these patients, and once initiated in the remodeled atria tends to be sustained. Thus ablation of the PV triggers alone may be less likely to render patients with long lasting persistent AF symptom free with a single procedure. Therefore additional left atrial ablation may be performed in some patients, depending on your electrophysiologists practice. Additional ablation targets include ablation of complex fractionated potentials (CAFÉ), linear lesions between the superior PVs or the left PV and mitral valve, or more extensive ablation of the posterior left atrial wall and septum. At UCSF, we favor a conservative approach to ablation focusing on broadly isolating the pulmonary veins. However, some of the above approaches may be employed depending on your AF.
This PV antral isolation procedure has been accepted at most centers as the standard for patients with paroxysmal AF. The need for repeat procedures may be higher in patients with more persistent forms of AF. Fibrosis, dilatation and other structural changes occur in the atrium after years of AF and tend to make AF more easily sustained. Premature atrial beats from areas outside the PVs may also initiate AF in these patients, and once initiated in the remodeled atria tends to be sustained. Thus ablation of the PV triggers alone may be less likely to render patients with long lasting persistent AF symptom free with a single procedure. Therefore additional left atrial ablation may be performed in some patients, depending on your electrophysiologists practice. Additional ablation targets include ablation of complex fractionated potentials (CAFÉ), linear lesions between the superior PVs or the left PV and mitral valve, or more extensive ablation of the posterior left atrial wall and septum. At UCSF, we favor a conservative approach to ablation focusing on broadly isolating the pulmonary veins. However, some of the above approaches may be employed depending on your AF.
